Featured Quizzes
User Quizzes
Create Quiz
Data and Charts
Badges and Games
About JetPunk
JetPunk Shop
Dark Mode
Valence Shell Electron Pair Repulsion (VSEPR) Theory
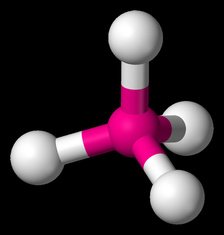
The Valence Shell Electron Pair Repulsion Theory (VSEPR) is the most widely accepted method to predict molecular geometries based on the interactions between electron pairs and how those interactions cause the electron pairs to arrange themselves. Click the molecular geometry that corresponds to the visual representation of the molecule.
In the AXE Visualization, A is the central atom, X is a bonded atom, and E is a lone electron pair.
Source: this article and my AP Chemistry knowledge
Save time by using Keyboard Shortcuts
Rate:
Last updated: March 10, 2023
You have not attempted this quiz yet.
More quiz info >>
| First submitted | March 10, 2023 |
| Times taken | 88 |
| Average score | 43.8% | Report this quiz | Report |
5:00
0
guessed
16 remaining
Time Used
00:00
Best Time
00:00
The quiz is paused. You have remaining.
Scoring
You scored / = %
This beats or equals
% of test takers
also scored 100%
The average score is
Your high score is
Your fastest time is
Keep scrolling down for answers and more stats ...





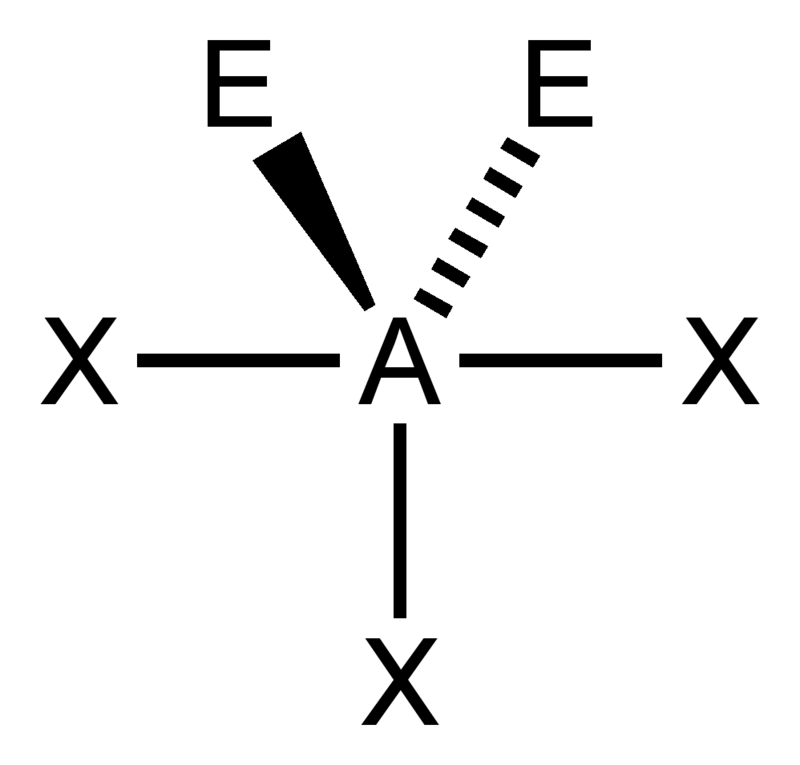



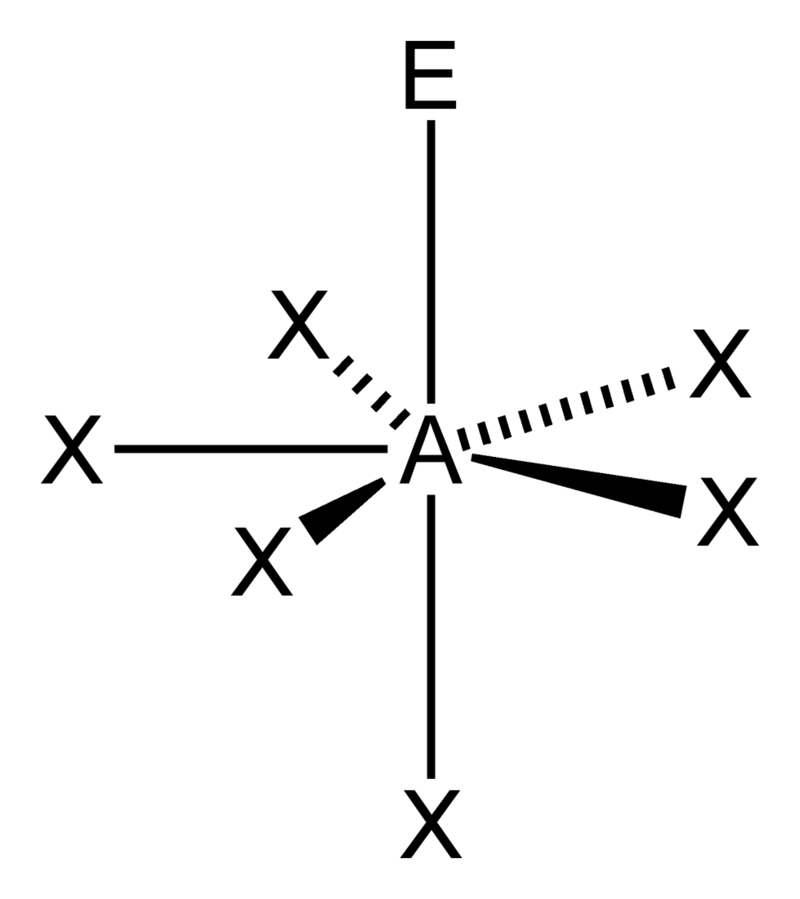





Bent (tetrahedral)
Bent (trigonal)
Linear (no lone pairs)
Linear (three lone pairs)
Octahedral
Pentagonal Bipyramidal
Pentagonal Planar
Pentagonal Pyramidal
Seesaw/Disphenoidal
Square Planar
Square Pyramidal
T-shaped
Tetrahedral
Trigonal Bipyramidal
Trigonal Planar
Trigonal Pyramidal
Correct!
Incorrect
You left this blank
Comments
No comments yet
New and Popular
Save Your Progress
Copyright H Brothers Inc, 2008–2024
Contact Us | Go To Top | View Mobile Site
